End to end handling of spontaneous reports, solicited and clinical trial reports, medico legal cases etc from triage and database entry including medical review.
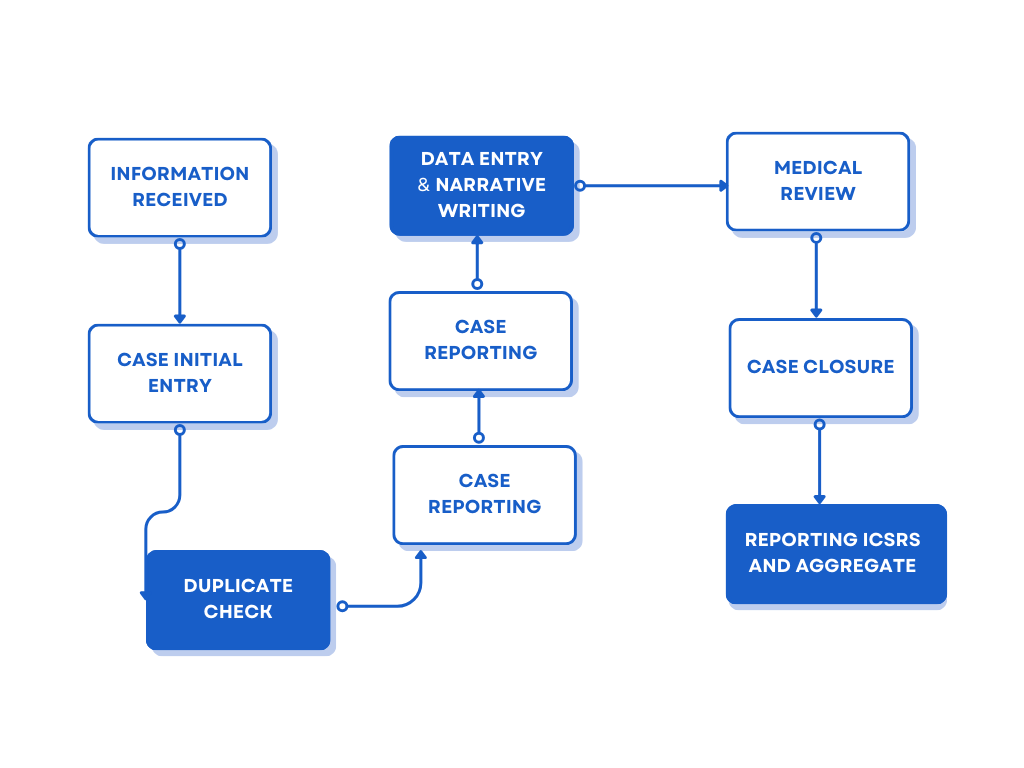
This service area covers the processing of individual case safety reports (ICSRs) originating from various sources:
Post-marketing non-solicited/Spontaneous reports
Clinical reports
Special reports (Medico-legal and Literature)
Related services
Data from source documents sent by clients and business partners is processed (data entry and MedDRA coding) in to a Drug Safety/Pharmacovigilance database (Arisg , Argus etc.) after a duplicate search, on behalf of the clients. This may involve an initial triage and a subsequent medical review/assessment of the report by physicians. Once finalized, the case reports are submitted to the regulatory authorities/business partners.